June 2025
France invests € 4M in Orixha’s reprocessing factory of breathable liquid for liquid ventilation
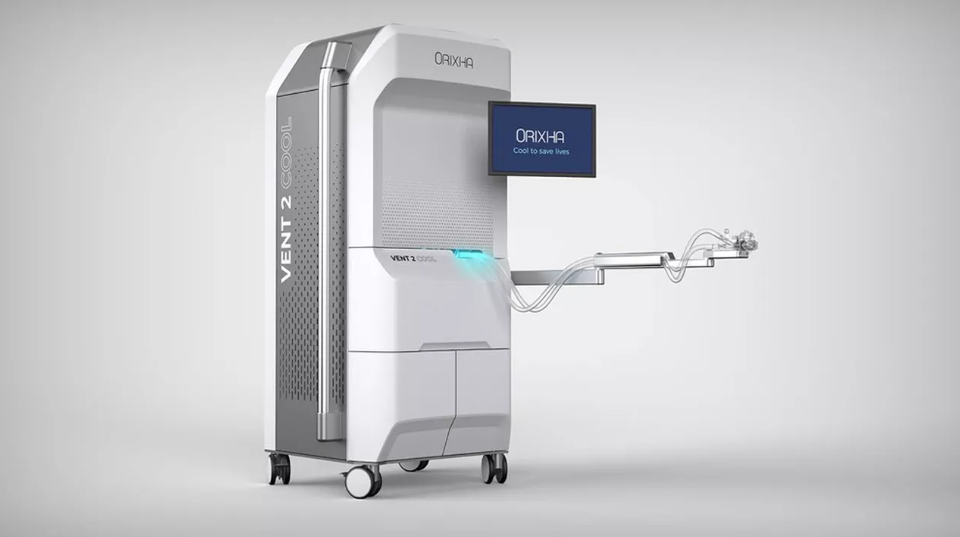
Lyon and Paris, May 20, 2025 – Orixha, a MedTech pioneer in liquid ventilation, announces that its (L2B)2 project for the industrial reprocessing of the L2B breathable liquid has been selected by the Première Usine program of France 2030 and will receive a grant plus loan award of 4 million euros. This financial support will enable Orixha to launch the scale-up and industrial phases of an exemplary reprocessing process in terms of technological and environmental innovation. Scheduled for commissioning in 2028 in the Plaine de l’Ain Industrial Park, this factory is essential to the commercialization of its Vent2Cool solution for resuscitated cardiac arrest patients.