LuncoLive: Orixha’s Proprietary TLV Technology
Developing a “Lung protective by design” Total Liquid Ventilation
The LuncoLive technology platform is, to date, the only total liquid ventilation solution that has been extensively validated in pre-clinical reference models. The OverCool clinical trial initiate clinical validation of LuncoLive technology in 2025.
TLV using LuncoLive technology is deemed to be “lung protective by design”. It ventilates the patient systematically under the residual functional capacity of the lungs thereby eliminating the risk of major lung injury such as perfluorothorax.
Through sensors, actuators, and algorithms, LuncoLive technology adapt in real-time the regulation of breathable liquid volume and pressure in the patients’ lungs. This patient-centric adaptative approach guarantees the safety of the procedure while preserving the physiology of the lungs.
The development of LuncoLive technology is supported by the following programs:
- i-Lab – Winner 2019; Grant of 245K€ from the French Ministry of Research,
- i-Nov – Laureate 2020; Grant and Loan from the Plan d’Investissement d’Avenir of 2.2M€ and,
- EIC Accelerator – Winner 2022; European Innovation Council grant of 2.4M€
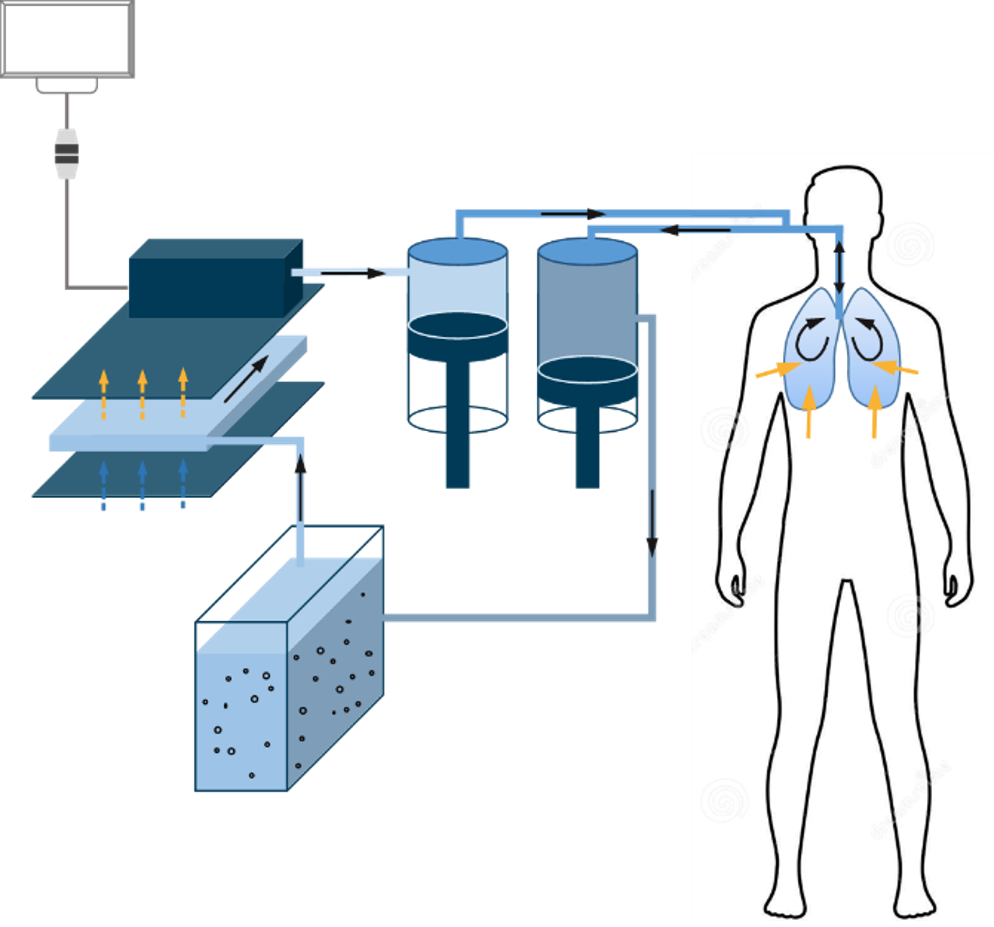
Principles of LuncoLive Total Liquid Ventilation
- Every 10 seconds, up to 1 liter of breathable liquid is pushed into the lungs of the comatose patient by an inspiratory pump through the endotracheal tubing
- In contact with the alveoli, the breathable liquid carries oxygen to the blood through capillaries and removes the carbon dioxide.
- The same quantity of breathable liquid is pumped out of the lungs by an expiratory pump.
- The Liquid Ventilator removes the CO2 from the breathable liquid and recharges it in oxygen.
- The Liquid Ventilator also regulates the temperature of the breathable liquid depending on the intended application: normothermia or hypothermia.
Orixha’s LuncoLive (acronym for Lung Conservative Liquid Ventilation) platform ambitions to become a new standard for Critical Care healthcare professionals and to help them answer unmet medical needs in post cardiac arrest syndrome, respiratory distress, and other life-threatening conditions.

How does TLV work ?
Total liquid ventilation (TLV) is an extracorporeal circulation of a breathable liquid that ventilates the lungs and provides respiratory support to intubated and comatose patients
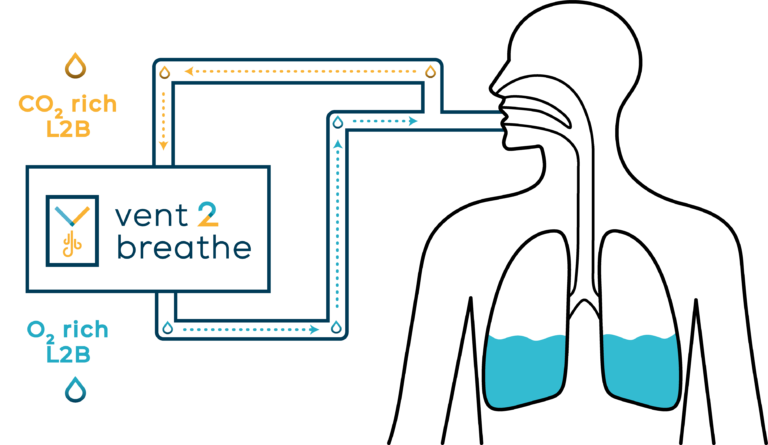

Hypothermic and Normothermic TLV
Temperature control of the Breathable Liquid is a key feature of TLV which can be delivered as Hypothermic or Normothermic TLV (i.e. liquid set at 36 – 37°C).
Vent2Cool, is a hypothermic TLV medical device that instills cool (20 to 33°C) breathable liquid and transforms the lungs into a heat exchanger with the blood compartment. This allows to induce an ultra-rapid therapeutic hypothermia in a few minutes where current hypothermia solutions take hours to reach the target temperature of 33°C.
Normothermic TLV incorporating LuncoLive technology holds great promise as a new respiratory support option for patients with Acute Respiratory Distress Syndrome.
The Orixha team is working on the development of a new medical device to provide respiratory support while avoiding the mechanical pro-inflammatory stress applied to the lungs with traditional mechanical ventilation. The future device, to be named Vent2Breathe, is currently in its initial development phase. Apre-clinical proof of concept on TLV for Respiratory Distress is underway and supported by the LiveResp ANR Research Program that includes EnvA and the Louis Mourier Hospital (AP-HP Paris) as research partners alongside Orixha.

Publications
- https://www.ahajournals.org/doi/10.1161/JAHA.124.035617 Ultrafast Cooling With Total Liquid Ventilation Mitigates Early Inflammatory Response and Offers Neuroprotection in a Porcine Model of Cardiac Arrest, 2024
- https://pubmed.ncbi.nlm.nih.gov/33198571/ Ultrafast Hypothermia Selectively Mitigates the Early Humoral Response After Cardiac Arrest, 2020
- https://pubmed.ncbi.nlm.nih.gov/31447395/ A new paradigm for lung-conservative total liquid ventilation, 2020
- https://pubmed.ncbi.nlm.nih.gov/26910322/ Liquid Ventilation for the Induction of Ultrafast Hypothermia in Resuscitation Sciences: A Review, 2016
- https://pubmed.ncbi.nlm.nih.gov/26878327/ Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation, 2016
- https://rea.revuesonline.com/2016/08.html Post-Cardiac Arrest Syndrome, 2016
- https://pubmed.ncbi.nlm.nih.gov/26110489/ Hypothermic Total Liquid Ventilation Is Highly Protective Through Cerebral Hemodynamic Preservation and Sepsis-Like Mitigation After Asphyxial Cardiac Arrest, 2015
- https://pubmed.ncbi.nlm.nih.gov/21810660/ Ultrafast and whole-body cooling with total liquid ventilation induces favorable neurological and cardiac outcomes after cardiac arrest in rabbits, 2011

