Vent2Cool for Post Cardiac Arrest Syndrome
The Vent2Cool Medical Device
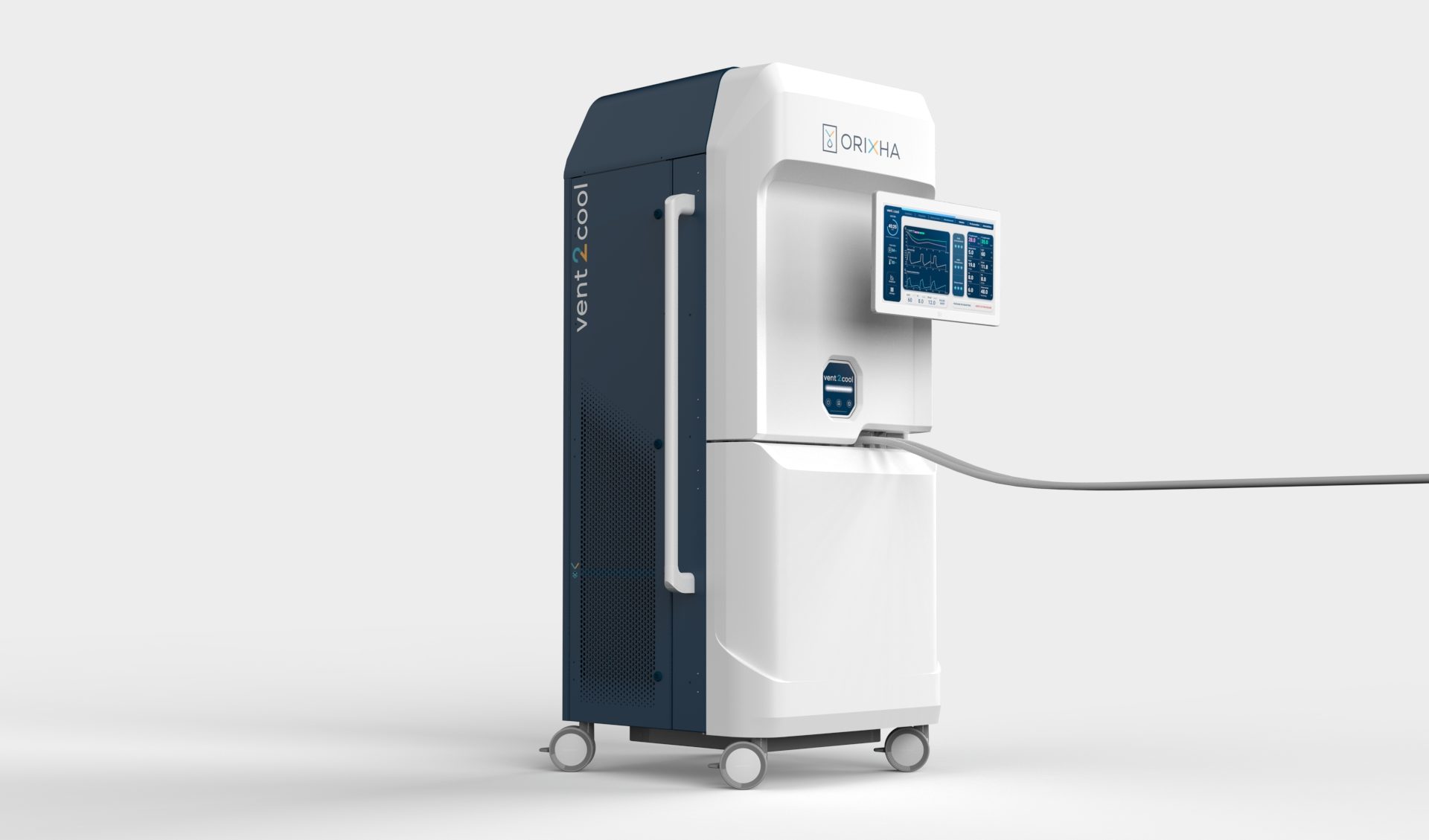
By adapting the LuncoLive platform to deliver hypothermic Total Liquid Ventilation, Orixha develops a disruptive medical device to induce ultra-rapid therapeutic hypothermia: Vent2Cool. Vent2Cool uses the lungs as thermal bio-exchangers to adjust precisely and continuously the blood and body temperature throughout the hypothermia induction procedure that lasts around 30 minutes.
Due to the unique exchange surface of the lungs with the blood compartment, the cooling power and speed to reach therapeutic hypothermia are orders of magnitude greater than with current hypothermia devices.
Vent2Cool is an experimental device, not yet approved for clinical use in any geography, designed to tackle post cardiac arrest syndrome in resuscitated cardiac arrest patient.
Vent2Cool in Action
As shown in the animation movie below, Vent2Cool was designed to complement and strengthen the OHCA survival chain through its unique capacity to induce protective ultra-rapid therapeutic hypothermia.

The Vent2Cool Product Development
Vent2Cool stems from over a decade of academic research and will be the first clinical application of the LuncoLive technology. The project capitalized on early works performed in the 2000’s at Université de Sherbrooke’s Inolivent Lab headed by Pr P. Micheau on normothermic liquid ventilation (prototypes Inolivent 1 to 3). Vent2Cool is the result of the development and validation of four prototypes (2 academic and 2 industrial) which have been validated for pre-clinical performance, tolerance, and impact in numerous relevant in-vivo models since 2011. This work has been the subject of 40 scientific publications. Experimentally, in Post Cardiac Arrest in-vivo models, Vent2Cool significantly improves survival outcomes by reducing drastically neurological sequels.


Cardiac Arrest
Out of Hospital Cardiac Arrest (OHCA) is a major Public Health issue. With an incidence of 6 for 10,000 in western countries and a survival rate around 10%, it strikes 700,000 adults and kills close to 650,000 persons every year in Europe and North America.
Emergency Medical Services with the help of bystanders manage to initially resuscitate successfully about a third of the patients and to transfer them comatose but alive to Cardiac Arrest Centers and Intensive Care Units.
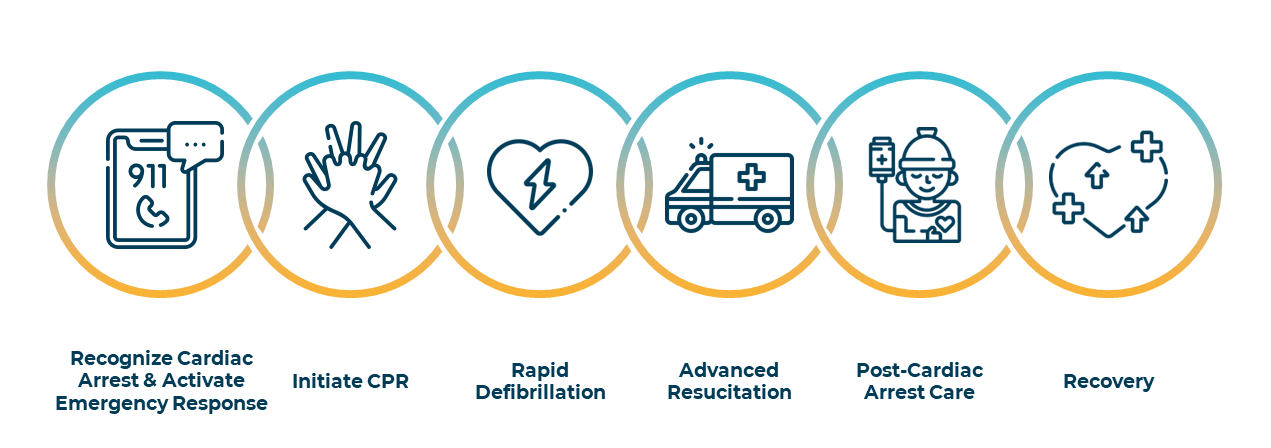
The Out of Hospital Cardiac Arrest Survival Chain approach to introduce best practices for all stakeholders as presented below is implemented in most countries. Investments in Cardio-Pulmonary Resuscitation trainings for bystanders, availability of defibrillators in public spaces and workspaces as well as location-based applications for cardiac arrest witnesses such as SauvLife in France are increasing the success rate in pre-hospital care for OHCA.
However, in-hospital care remains the weakest link. Therefore, unfortunately, around two-third of these initially resuscitated patients die in the hospital within a few hours from brain, heart, or multi-organ failure, triggered by a massive systemic cytokine storm called Post Cardiac Arrest Syndrome. Consequently, survival rate with good neurological recovery from out of hospital cardiac arrest is under 10% in the western world.
Orixha is dedicated to change the game with ultra-rapid hypothermia induction delivered by Vent2Cool for resuscitated cardiac arrest patients.

Therapeutic Hypothermia
To date, the way to fight Post Cardiac Arrest Syndrome and thereby to reduce in-hospital mortality of cardiac arrest is through therapeutic hypothermia.
This should be done as quickly as possible post the initial resuscitation when the heart restarts thanks to a defibrillator and / or CPR. Therapeutic hypothermia works by protecting critical organs from the Post Cardiac Arrest Syndrome at a temperature of 32 – 33°C. As shown by academic teams working on cardiac arrest physiopathology, ultra-rapid hypothermia induction reduces the inflammatory cytokine storm and lowers the cellular metabolism of neurons and other critical organs. Consequently, it protects the brain, heart and kidneys from the Post Cardiac Arrest cytokine storm.
Vent2Cool cools down the heart and brain to a protective 33°C in less than 15 minutes vs. several hours for current hypothermia solutions.

Clinical development plan
Vent2Cool is an experimental device that will be subject to extensive clinical validation before its approval by regulatory authorities, leading to its clinical use in cardiac arrest centers and ICUs worldwide. The OverCool (NCT06798818) pilot clinical trial, whose protocol has been published, has been initiated in 2025 in two leading Cardiac Arrest Centers :
- Cochin Hospital (AP-HP, Paris France) – Lead Investigator Pr Alain Cariou
- CHU Angers (Angers France) – Lead Investigator Pr Alain Mercat
The clinical validation of V2C is financially supported by the i-Nov programme and the EIC Accelerator with the Luncolive project.
Further clinical studies are being planned in collaboration with Orixha Scientific Committee and will aim to demonstrate the superior clinical benefits brought by Vent2Cool’s ultra-rapid hypothermia induction when added to current Targeted Temperature Management solutions.